
The Women’s Health Research Institute (WHRI) in partnership with the BC Women’s Health Foundation is pleased to congratulate the recipients of the first Perinatal Reseach Imaging and Evaluation (PRIME) Centre Research Award.
The PRIME Centre, located in the Skidmore Goodman Research Centre for Women’s Health at BC Women’s Hospital + Health Centre, is a state-of-the-art ultrasound imaging research facility. Its mission is to develop new imaging technology to improve maternal, fetal, and neonatal health. The centre provides researchers with access to cutting-edge equipment and offers opportunities for training in ultrasound technical skills.
A total of 2 awards were distributed with a value of $11,250 each. The PRIME Centre Awards provide funding for research studies focused on women’s and newborn health. They must be conducted in the PRIME Centre and take advantage of the available equipment, personnel support, and facility features, while emphasizing collaborative projects between university departments, medical specialties, and industry in accordance with CFI guidelines.
Recipients

PROJECT TITLE:
Placenta, FEtal Brain BLood flow, and Substance Use ExposureS (PEBBLES)
PRINCIPAL INVESTIGATOR:
Title: Medical Director, Perinatal Addictions, BCWH; Clinical Associate Professor
Department: Family Practice
Tim Oberlander, Wendy Robinson, Ken Lim, Jeff Bone, and Robert Rohling
SUMMARY:
Canada is facing a growing opioid epidemic, and women are being prescribed opioid agonist therapy (OAT) like methadone at higher rates. However, little is known about the effects of OAT on a pregnant person’s placenta (important to baby’s health) or baby. Women taking OAT in pregnancy admitted to the FIR unit at BC Women’s Hospital will be invited to take part in a survey and two 1-hour ultrasounds at the Perinatal Research IMaging Evaluation (PRIME) Centre. Placenta and baby will be evaluated on ultrasound before, and after a participant takes their regular prescribed OAT, to learn about the placenta and baby’s health in the presence of OAT.
Women and gender-diverse birthing people who use substances in pregnancy are significantly underrepresented in research. The PEBBLES team, in collaboration and partnership with persons with lived and living experience (PWLLE), aims to inform clinicians on opportunities to tailor pharmacologic management in pregnancy, and ultimately improve the care and treatment of women with opioid use disorders and their infants.

PROJECT TITLE:
The Facial Profile Angle: A pilot project to improve prenatal detection of retrognathia and micrognathia.
PRINCIPAL INVESTIGATOR:
Dr. Denise Pugash, CFI Grant Award recipient, the Skidmore Goodman PRIME Research Centre, BC Women’s Hospital & Health Centre,
Clinical Professor, Department of Radiology
Nick Garbuz, Amanda Easton, and Kirsten Grabowska
SuMMARY:
The presence of a severely small chin (micrognathia) in a baby may lead to serious and life-threatening conditions. The milder form, retrognathia, can be seen with syndromes and other significant abnormalities. The prenatal ultrasound diagnosis of both conditions is typically based on a subjective impression by the person responsible for the ultrasound report. The existing published methods of measuring chin size are cumbersome and inaccurate. There is no existing method to determine the difference between retrognathia and micrognathia, which would help to plan clinical management.
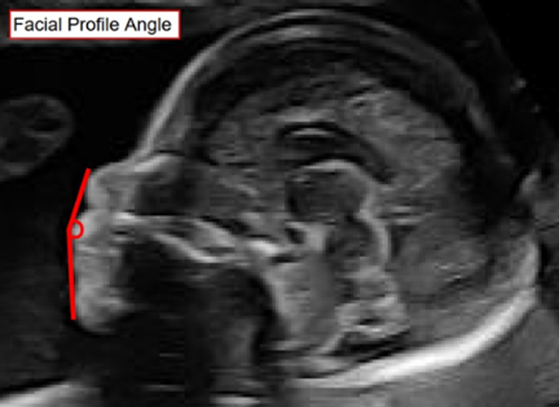
We aim to assess the feasibility of a novel simple measurement, the “Facial Profile Angle” (FPA), which uses 2D and/or 3D ultrasound scans to measure the angle between the tip of the nose, the upper lip, and the chin.
Using fetal images of the facial profile, the FPA technique will serve as a simple, objective, and reproducible screening tool to help differentiate between normal chin size and retrognathia or micrognathia. We hypothesize that a normal range of values can be obtained using the FPA. These values will be compared with images in babies with confirmed abnormalities of chin size. We aim to obtain a consistent and reliable measurement which avoids false positive diagnoses. Additionally, identifying fetuses with true retrognathia od micrognathia early in pregnancy will help to identify newborns that require extra support at delivery. This will ensure that healthcare providers can work with the family to establish a plan for the newborn and will allow the best possible healthcare outcomes for the child.