Back to all News & Events >
Author Archives: WHRI

BC’s First Menopause Study Highlights Midlife Women’s Experiences with Menopause
Health and Economics Research on Midlife Women in British Columbia On November 1, 2024, the Health and Economics Research on Midlife Women in British Columbia (HER-BC) report was released at the Greater Vancouver Board of Trade Healthcare Forum. The study, conducted in partnership with the BC Women’s Health Foundation and Pacific Blue Cross, recruited over 2,000 […]

The Beyond the Binary Canada Knowledge Exchange 2024
Launching the new, national, guide for the research community On October 16th, 2024, the Partnership for Women’s Health Research Canada (PWHR) in collaboration with the Women’s Health Research Institute (WHRI) hosted the Beyond the Binary National Knowledge Exchange. This event brought together more than 200 attendees including trainees, researchers, as well as representatives from government and […]

Digital Health Week 2024
Celebrating Digital Health Week Let’s Celebrate Digital Health! Digital Health Week, November 18th – 24th, is the annual celebration and recognition of how digital health is transforming the delivery of care across Canada. Have you heard about the WHRI/BCCHR Digital Health Research Program? We are a joint program being led collaboratively by the BC Children’s […]

WHRI & BC Children’s Hospital BioBank Testimonial – Dr. Pascal Lavoie Preemie Biobank
Neonatologist Dr. Pascal Lavoie’s recently published research could eventually lead to therapeutic interventions for sepsis, lung disease, and other complications in premature newborns. We were able to connect with him and the team about the Preemie Biobank samples used to in his research, and how the Preemie Biobank is a valuable resource for other researchers […]
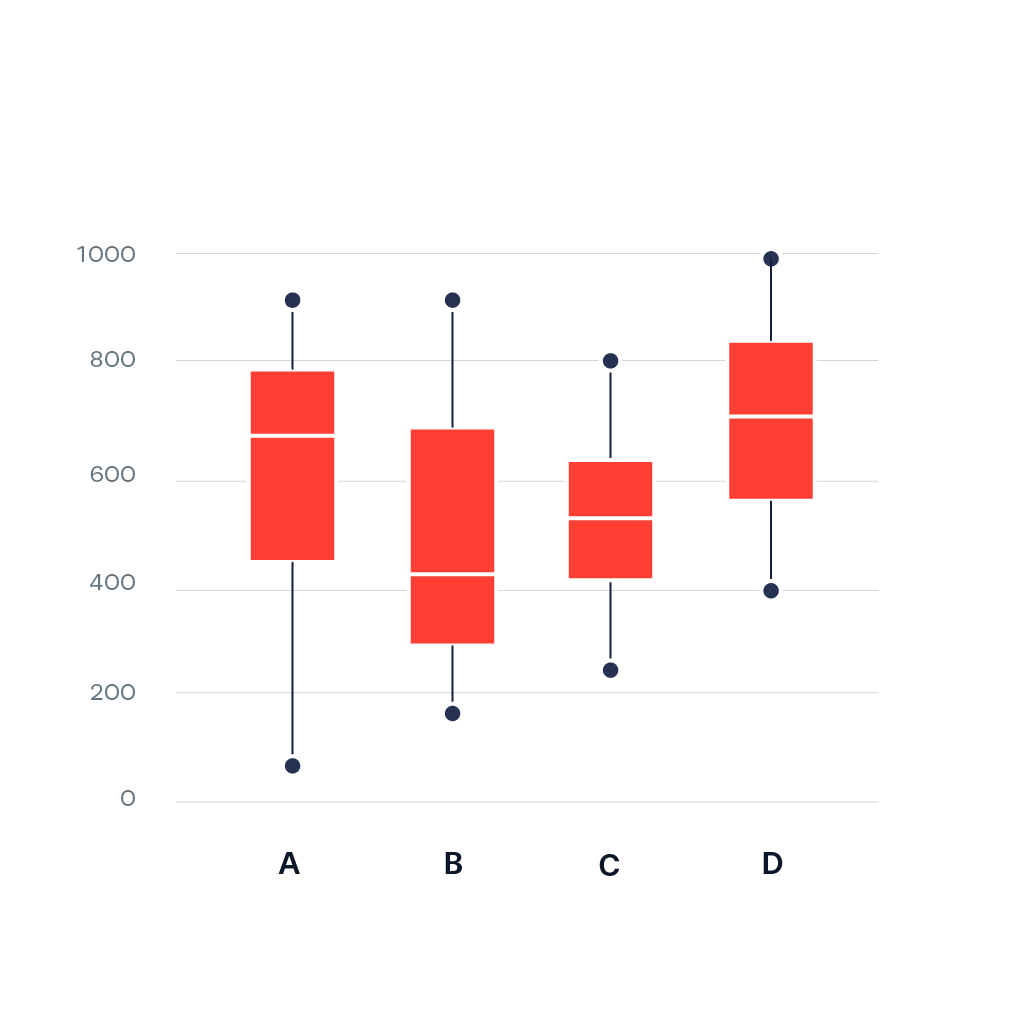
Sabina’s Stats Corner: Understanding Box Plots: A Comprehensive Guide Part 2
Special Notes: Listen to our L&L lectures online: WHRI Lunch & Learn Series – Women’s Health Research Institute Visit our Stats corner in the e-blast for previously published tips on data management and analysis: E-Blast Archive – Women’s Health Research Institute (whri.org) Check out our new YouTube channel for insightful presentations and resources: WHRI Communications – YouTube […]

Member in the Media: Dr. Ruth Elwood Martin
WHRI member Dr. Ruth Elwood Martin’s career as a family physician in Vancouver took a transformative path when a part-time role in prison medicine changed her life. Her innovative participatory research has greatly improved the well-being of incarcerated women and their infants.

A Recap of World Sexual Health Day 2024
Celebrating World Sexual Health Day 2024: Positive Relationships On September 4th, the WHRI, in partnership with Options for Sexual Health (OPTIONS) and the World Association for Sexual Health (WAS), celebrated World Sexual Health Day (WSHD) and aimed to dismantle social and cultural taboos surrounding sexuality while advocating for positive sexual health worldwide. The theme for WSHD 2024 […]

2024 Graduate and Fellowship Research Award in Women’s Health Recipients
The Women’s Health Research Institute (WHRI) is very pleased to congratulate the recipients of the 2024 Graduate and Fellowship Research Awards in Women’s Health. This competition was supported thanks to the dedicated funding provided by the BC Women’s Health Foundation. The WHRI launched this award in 2020 with a goal of creating a funding opportunity […]

2024 Dr. Monica K. Li and Family Undergraduate Medical Student Research Award in Women’s Health Recipient
The Women’s Health Research Institute (WHRI) is very pleased to congratulate the recipient of the inaugural Dr. Monica K. Li and Family Undergraduate Medical Student Research Award in Women’s Health. Thanks to an annual endowment by Dr. Monica K. Li and family to the BC Women’s Health Foundation, the WHRI has established the Dr. Monica […]